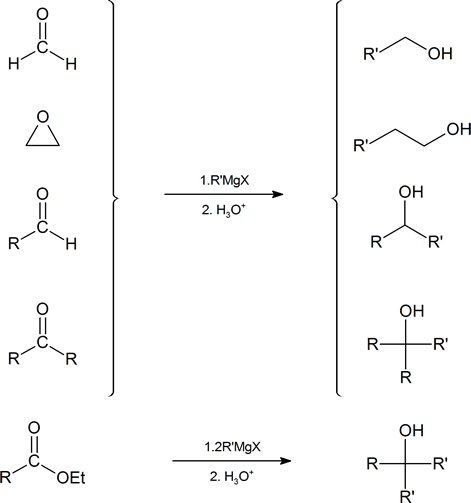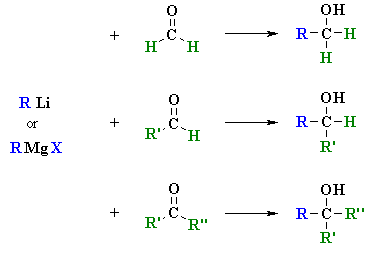Synthesis Of Vinyl Grignard Reagent
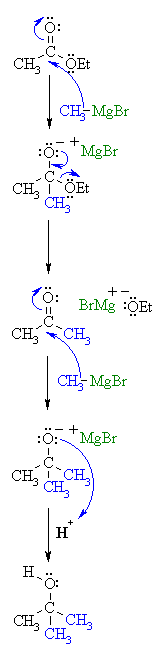
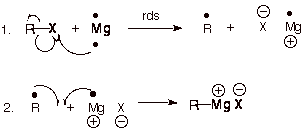
See gallery the grignard reaction pronounced ɡriɲar is an organometallic chemical reaction in which alkyl allyl vinyl or aryl magnesium halides grignard reagent add to a carbonyl group in an aldehyde or ketone.
Synthesis of vinyl grignard reagent. Bulletin of the chemical society of japan 1964 37 2 207 209. These alkyl vinyl or aryl magnesium halides are referred to as grignard reagents. Industrially the grignard reaction is the key step in the production of tamoxifen which is used in the treatment of breast cancer. The reaction is considered an important tool to form carbon carbon bonds.
A solution of a carbonyl compound is added to a grignard reagent. The alkynyl grignard reagents are prepared by deprotonating 1 alkynes with another grignard reagent like ethylmagnesium bromide. The synthesis of β ionylideneacetaldehyde. Vinyl bromide and bromobenzene can be converted to corresponding grignard reagents by reacting them with magnesium metal in anhydrous thf.
The reaction is often unsuccessful without substitution ortho to the nitro group with bulkier ortho substituents usually resulting in higher. Canadian journal of chemistry 2008 86 3 213 218. Grignard reaction mechanism explains the addition of alkyl vinyl aryl magnesium halides to any carbonyl group in an aldehyde ketone. Grignard reagents form easily from 1 2 and 3 alkyl halides although their reactivities differ.
A new approach to the synthesis of 7 substituted indoles giuseppe bartoli gianni palmieri dipartimento scienze chimiche via s agostino 1 62032 camerino mc italy marcella bosco renato dalpozzo. Currently grignard degradation is used for the chemical analysis of certain triacylglycerols. The synthesis of the grignard reagent occurs on the. Prodigiosin synthesis with electron rich 2 2 bipyrroles.
Grignard reagent can be used for determining the number of halogen atoms present in a halogen compound. Aryl and vinyl halides react somewhat more slowly and the cyclic ether tetrahydrofuran thf is required to prepare grignard reagents of these compounds. The bartoli indole synthesis also called the bartoli reaction is the chemical reaction of ortho substituted nitro arenes and nitroso arenes with vinyl grignard reagents to form substituted indoles.