Synthesis Of Vinyl Azides

2004 888 doi.
Synthesis of vinyl azides. Keywords 2h azirine nitrene nitrogen heterocycles synthon vinyl azides α azidovinylketone. Azides are highly versatile organic functional groups and their preparation and their reactivity are well explored in contrast the synthesis of vinyl azides is far away from being well established despite the fact that an even richer chemistry than that which has lately been developed for azides can be envisaged as vinyl azides bear the additional alkene moiety. C n bond formation synthesis of azides synthesis of alkyl azides. Since the discovery of phenyl azides by peter grieβ in 1864 1 numerous novel transformations of azide derivatives have emerged as rapid and versatile methods for the synthesis of a variety of complex n heterocyclic systems.
After optimizing the elimination cyclization reaction of e β phenyl vinyl bromide with phenyl azide we attempted to employ similar reaction conditions to synthesize 1 5 diphenyl 1 2 3 triazole from α phenyl vinyl bromide and phenyl azide. Synthesis of aryl azides and vinyl azides via proline promoted cui catalyzed coupling reactions w. A practical rapid and efficient microwave mw promoted nucleophilic substitution of alkyl halides or tosylates with alkali azides thiocyanates or sulfinates in aqueous media tolerates various reactive functional groups. And the results are summarized in table 2 in general compared to e β phenyl vinyl bromide α phenyl vinyl bromide exhibited a different reaction.
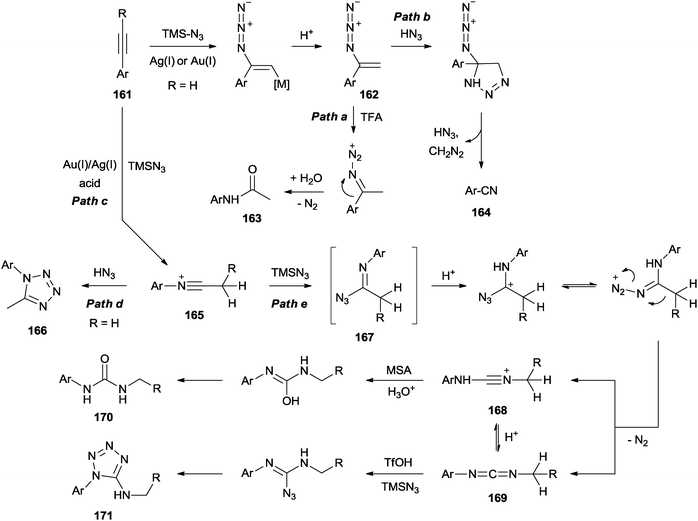
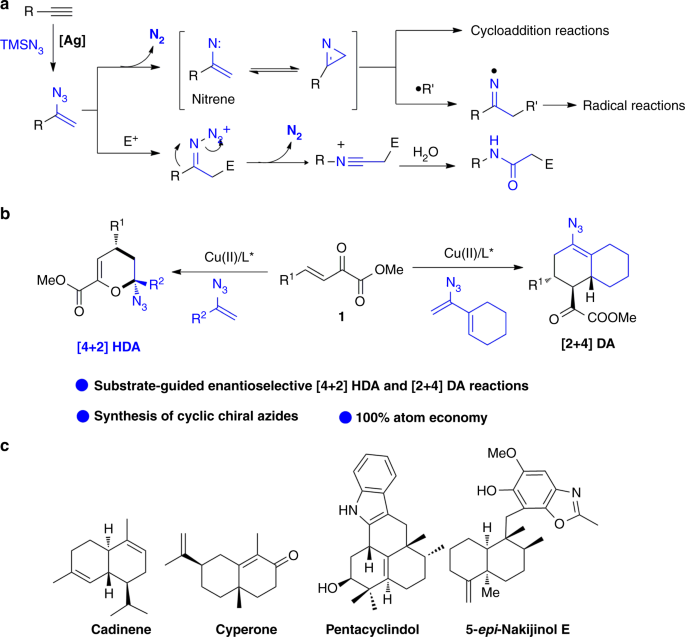
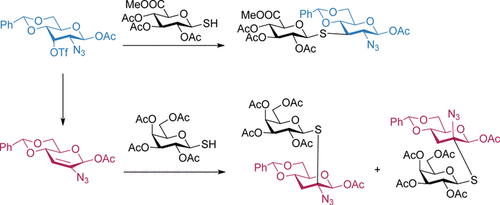
This synopsis highlights and discusses recent advances on use of vinyl azides in chemical synthesis as a radical acceptor and an enamine type nucleophile. A divergent synthesis of amides and nitriles from vinyl azides and p quinone methides p qms was developed. 2 however vinyl azides have been regarded as sleepers among the reactive azido species and they drew little attention over the course of the last. The nucleophilic addition of sodium azide to 1 2 allenyl esters regio and stereoselectively generates vinyl azides in excellent yields.
The p qms could be activated by bf3 et2o and then nucleophilically attacked by vinyl azides leading to divergent rearrangement toward amides and nitriles. Title vinyl azides as versatile synthons for the synthesis of nitrogen containing heterocycles volume. 9 author s hangcheng ni guolin zhang and yongping yu affiliation college of pharmaceutical sciences zhejiang university hangzhou 310058 p.